Newsroom
Get in touch and get the news
Welcome to our resources for media coverage of Intuitive and its products. Get in touch with us and see regional press releases. See company statements and a library of Intuitive image and video assets that you can use for promotional, editorial, and academic use.
Media enquiries
UK and Ireland: +44 7385 684197 (8am to 6pm GMT).
Other countries outside the US: +41 21 821 2000 (8am to 6pm CET).
Within the US: +1 408-523-8181 ext. 6934681
Intuitive press releases
For the UK and Ireland, see the following press releases highlighting the latest regional news.
- December 2025: Ion robotic-assisted bronchoscopy could enable early lung cancer diagnosis, UK-first study finds
- October 2025: Intuitive opens UK’s largest robotic-assisted surgery training centre
- July 2025: da Vinci 5 receives CE Mark
- January 2025: Major robotic-assisted surgery milestone for Scotland
- September 2024: UK First Robotic-Assisted Day Case Surgery Programme
- January 2024: Da Vinci Single-Port Surgical System Receives CE Mark for Multiple Procedures
- August 2023: UK First Robotic-Assisted Lung Biopsy Procedures Performed
- July 2023: RCS England collaborates with Intuitive to supercharge robotic surgery
- June 2023: Intuitive and Newcastle Surgical Training Centre collaborate on UK’s first robotic-assisted surgery training programme
- October 2022: Intuitive becomes largest robotic-assisted surgery provider to be accredited by Royal College of Surgeons of England
- September 2022: Intuitive donates early version of its da Vinci surgical system to Royal College of Surgeons of England
Corporate overview
Learn about Intuitive’s company history and product evolution in our fact sheet that includes timelines and statistics describing our global business.
Company Statement
Intuitive (Nasdaq: ISRG), headquartered in Sunnyvale, California, is a global leader in minimally invasive care and the pioneer of robotic surgery. Our technologies include the da Vinci surgical system and the Ion endoluminal system. By uniting advanced systems, progressive learning, and value-enhancing services, we help physicians and their teams optimize care delivery to support the best outcomes possible. At Intuitive, we envision a future of care that is less invasive and profoundly better, where diseases are identified early and treated quickly, so patients can get back to what matters most.
Image and video library
These materials are provided exclusively for promotional, editorial, and academic use and/or for use in media coverage of Intuitive and its products. Please download zip files that have been packaged to deliver a set of product images or logo files for you to use in your publication.
Terms of use
Intuitive provides these materials without warranty of any kind. Therefore, please note that permission to use them is only granted if the following guidelines are observed.
While Intuitive authorizes your use of these materials as appropriate, Intuitive promotes and advertises only for procedures cleared by applicable regulatory agencies. Any use of these materials by a third party is in the sole control and responsibility of that party for their promotional, editorial, and academic use and/or media coverage. Intuitive does not advise or provide input to customers on their marketing efforts or materials. You shall not include any statements in your use of these materials that implies that Intuitive assisted in creating or endorses the end product in which you have used these assets.
No material found in this section can be used to promote a procedure outside of a system or instrument’s approved/cleared Indications for Use.
Please refer to the product’s labeling for a description of clinical uses for which the da Vinci surgical system has been approved/cleared for use.
All people depicted are models unless otherwise noted.
Additional guidelines:
- Unless otherwise noted, permission is given to use these materials in their entire unmodified form
- Permission is not granted if these materials are modified in any way
- Images must be reproduced in high resolution
- Intuitive logos must be used in accordance with the Intuitive logo guidelines which can be downloaded under the Intuitive logos section below on this page
- Reproduced images should be accompanied by the following copyright notice: ©[Year] Intuitive Surgical Operations, Inc.
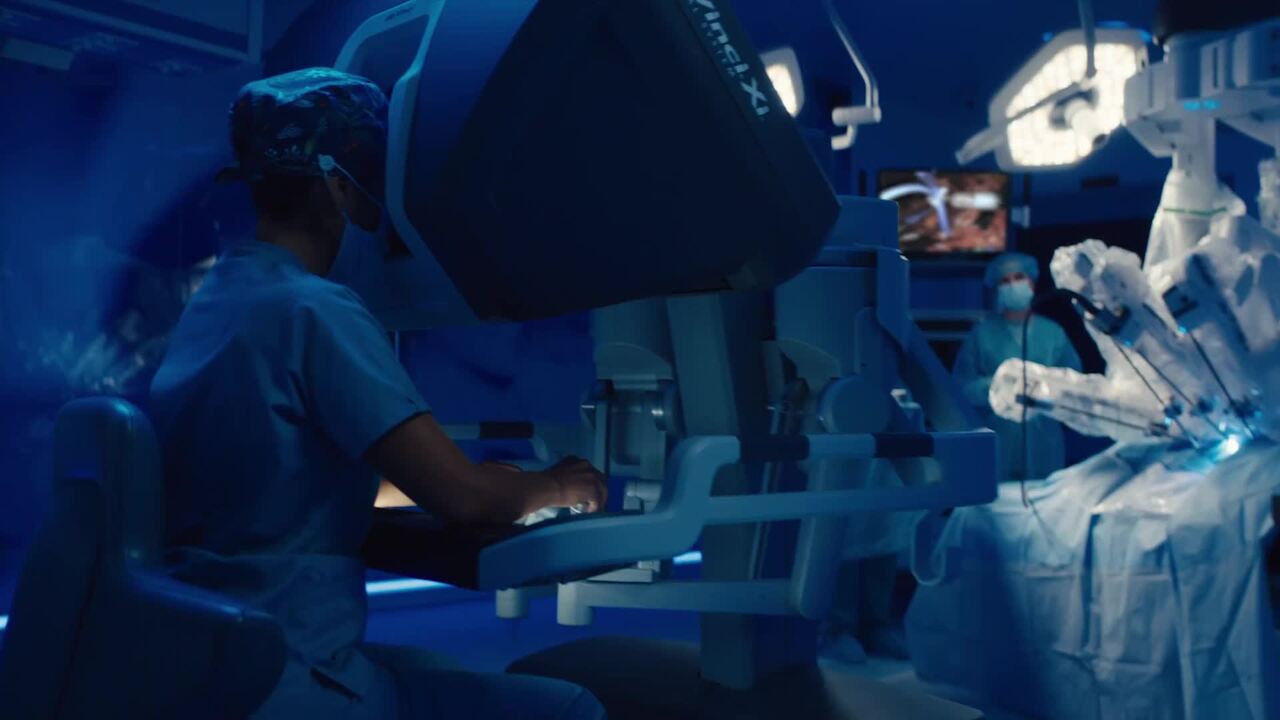
Videos: company and product
Use these links to download video files. Below find playback of each video to show you what is included—you may watch the videos in their entirety.


Legal Notices
Individuals' outcomes may depend on a number of factors, including but not limited to patient characteristics, disease characteristics and/or physician/surgeon experience.
Some products, features or technologies may not be available in all countries. Please contact your local Intuitive representative for product availability in your region. Refer to the product specific User Manual for indications, contraindications, warnings and other product information.
European Privacy Notice