Intuitive for Thoracic Surgeons
Advancing surgical care for your patients
Flexibility with the da Vinci Xi surgical system
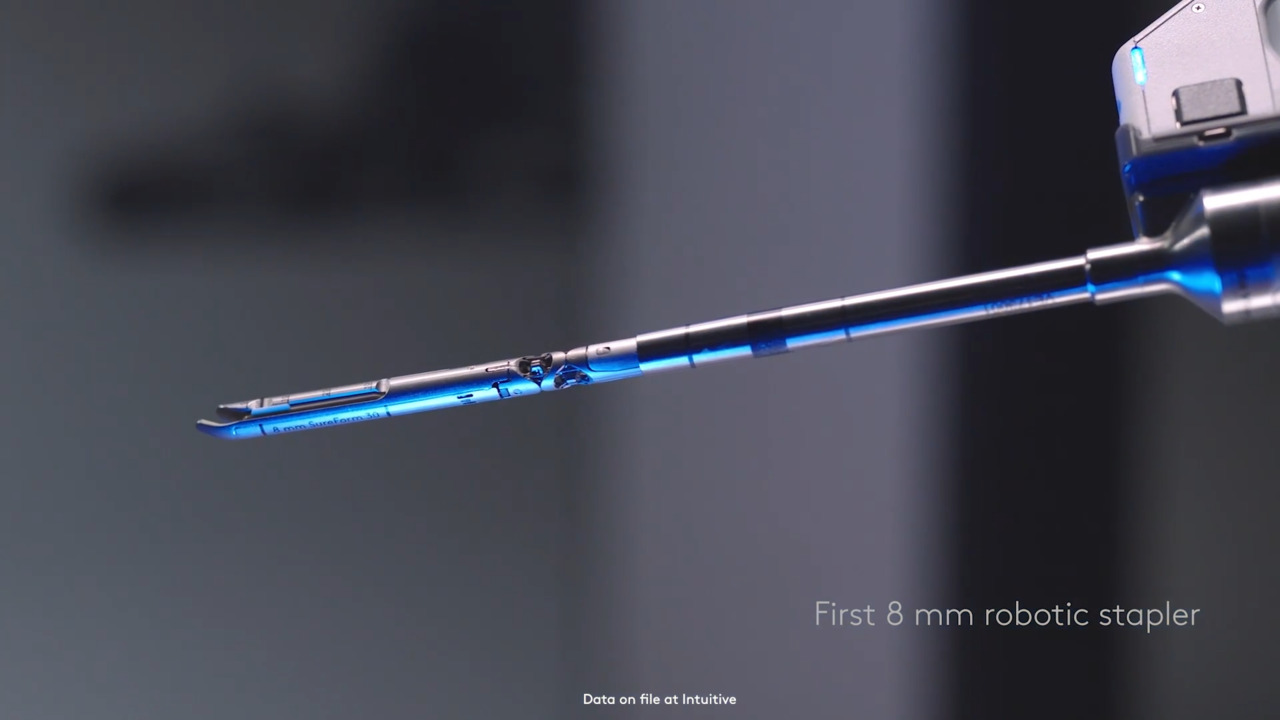
Da Vinci SureForm staplers
Staple with complete control, full articulation, and intelligent feedback. At SureForm’s core is SmartFire technology which monitors tissue compression before and during firing. A 120° cone of articulation gives you the freedom to approach tissue where it naturally lies, potentially reducing the need to pull it towards the stapler.1


Thoracic Connect: peer-to-peer education in an interactive setting
Thoracic Connect: peer-to-peer education in an interactive setting
Intuitive brings thoracic surgeons together at the EU Thoracic Connect Up event. Engage with peers, connect with thought leaders, and share the latest insights in the fast-changing world of minimally invasive lung cancer care with the da Vinci surgical system. Mentors in robotic-assisted surgery will share how they build a da Vinci program and utilize the systems across their thoracic practices by using the latest techniques and technology.
Resources
Resources
PORTal study points to better outcomes
Real-world comparative evidence shows fewer complications and shorter hospital stays for robotic-assisted lobectomies
1. Results from testing on file.
2. Compared to 12 mm SureForm staplers. Data on file at Intuitive.
Disclosures
Da Vinci X & Xi Surgical Systems
The Intuitive Surgical Endoscopic Instrument Control Systems (da Vinci X and da Vinci Xi Surgical Systems) are intended to assist in the accurate control of Intuitive Surgical Endoscopic Instruments during urologic surgical procedures, general laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, general thoracoscopic surgical procedures, and trans-oral otolaryngology surgical procedures restricted to benign tumors and malignant tumors classified as T1 and T2, and for benign base of tongue resection procedures. The systems are indicated for adult and pediatric use (except for trans-oral otolaryngology surgical procedures). They are intended to be used by trained physicians in an operating room environment.
The da Vinci X and da Vinci Xi Surgical Systems are class IIb medical devices CE marked (CE 2460) under the European Medical Devices Directive (93/42/EEC), manufactured by Intuitive Surgical, Inc. Refer to Instructions For Use before use.
SureForm 45 & 60 Staplers
The SureForm 45 Stapler & SureForm 60 Stapler and SureForm 45 Stapler Reloads & SureForm 60 Stapler Reloads and other Stapler Accessories (including the bladeless obturators) are intended to be used with a compatible da Vinci Surgical System for resection, transection, and, or creation of anastomoses in General, Thoracic, Gynecologic, Urologic, and Pediatric surgery. The devices can be used with staple line or tissue buttressing material (natural or synthetic).
The SureForm 60 Stapler and SureForm 45 Stapler, SureForm 60 and SureForm 45 Stapler Reloads are class IIa and IIb medical devices CE marked (CE 2460) under the European Medical Devices Directive (93/42/EEC), manufactured by Intuitive Surgical, Inc. Refer to Instructions For Use before use.
8 mm SureForm 30 Stapler Curved-Tip
The Intuitive Surgical 8 mm SureForm 30 Curved-Tip Stapler and 8 mm SureForm 30 Reloads and accessories are intended to be used with a compatible da Vinci Surgical System for resection, transection of vasculature and tissue, and/or creation of anastomoses in General, Thoracic, Gynecologic, Urologic, and Pediatric Surgery. The Intuitive Surgical 8 mm SureForm 30 Curved-Tip Stapler and 8 mm SureForm 30 Reloads and accessories are class IIa and IIb medical devices CE marked (CE 2460) under the European Medical Devices Regulation (EU 2017/745), manufactured by Intuitive Surgical, Inc. Refer to Instructions For Use before use.
Legal Notices
Individuals' outcomes may depend on a number of factors, including but not limited to patient characteristics, disease characteristics and/or physician/surgeon experience.
Some products, features or technologies may not be available in all countries. Please contact your local Intuitive representative for product availability in your region. Refer to the product specific User Manual for indications, contraindications, warnings and other product information.